

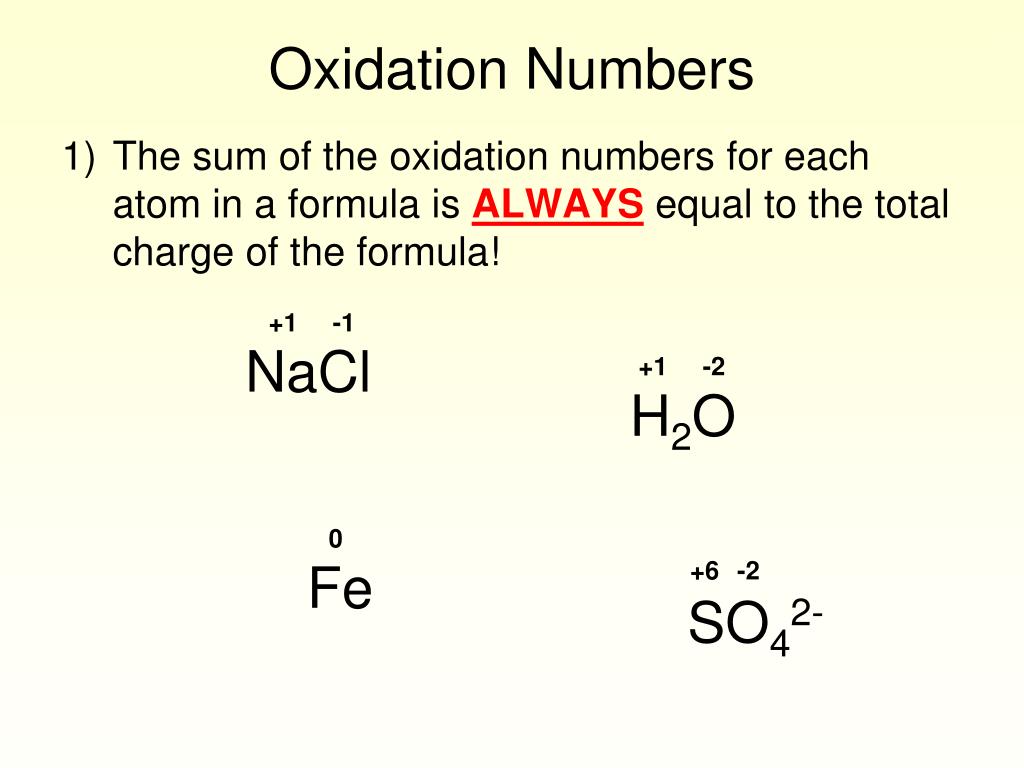
Ionic Compounds But since this ion has a charge of -2, the oxidation numbers must add up to negative two. Ionic Compounds Example: We don’t know chromium’s oxidation number from the periodic table since it is a transition metal.

Therefore, Fe has a +3 oxidation number in this compound. Ionic Compounds But since we know the compound is neutral, the oxidation numbers must add up to zero.
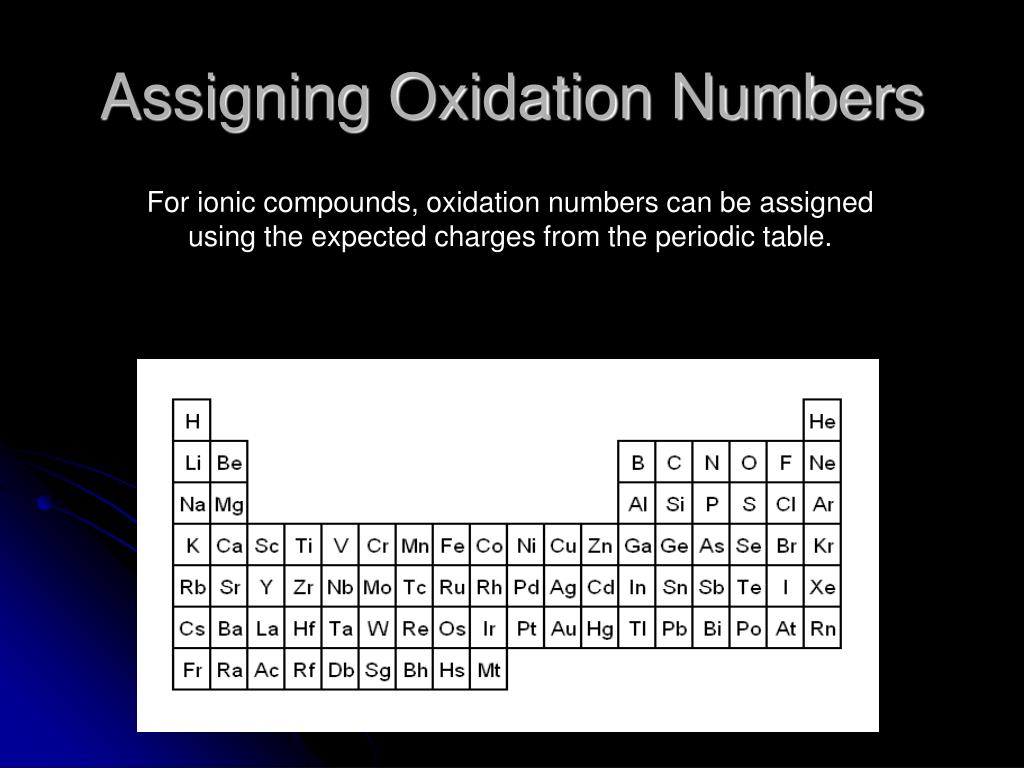
Ionic Compounds Example: We don’t know iron’s oxidation number from the periodic table since it is a transition metal. Halogens = -1Īssigning Oxidation Numbers For ionic compounds, oxidation numbers can be assigned using the expected charges from the periodic table. Group 16 Nonmetals = -2Īssigning Oxidation Numbers For ionic compounds, oxidation numbers can be assigned using the expected charges from the periodic table.
Group 15 Nonmetals = -3Īssigning Oxidation Numbers For ionic compounds, oxidation numbers can be assigned using the expected charges from the periodic table. Group 13 Boron Group = +3Īssigning Oxidation Numbers For ionic compounds, oxidation numbers can be assigned using the expected charges from the periodic table. Alkaline Earth Metals = +2Īssigning Oxidation Numbers For ionic compounds, oxidation numbers can be assigned using the expected charges from the periodic table. Alkali Metals = +1Īssigning Oxidation Numbers For ionic compounds, oxidation numbers can be assigned using the expected charges from the periodic table. Assigning Oxidation Numbers For ionic compounds, oxidation numbers can be assigned using the expected charges from the periodic table.Īssigning Oxidation Numbers For ionic compounds, oxidation numbers can be assigned using the expected charges from the periodic table.


 0 kommentar(er)
0 kommentar(er)
